Author Archives: UMassTox
As the opioid crisis continues, other health consequences of drug use are on the rise. Since 2016, hepatitis A has caused more than 230 deaths. As of August, 2019, there were 494 cases of hepatitis A in Massachusetts (and seven deaths) since the onset of the local outbreak in April of 2018.
Individuals at highest risk for hepatitis A include people who:
- Use drugs (injection or other routes)
- Are experiencing unstable housing or homelessness
- Are in prison or were recently released
- Have chronic liver disease (like hepatitis B, hepatitis C or cirrhosis)
Fortunately, a single dose of the hepatitis A vaccine can prevent hepatitis A and help control outbreaks. If you, or someone you know is at risk for hepatitis A, talk to your doctor about the vaccine.

Detergent pods (self-contained, single use packages of detergent for washing machines or dishwashers) have been on the market in the U.S. since 2010. Not long after their introduction, they became the frequent subject of calls to Poison Control Centers due to inadvertent ingestion by children under 6 years old. Given their size and brightly colored appearance, it’s easy to see how a child may mistake a detergent pod for a piece of candy. As many as 6,219 calls to Poison Control Centers in the U.S. in 2011 involved pediatric ingestion of detergent pods. Many children suffered only mild toxicity such as nausea and vomiting, but there are numerous reports of more serious events.
The chemicals in the membranes of these products can break down in to substances that can act like alcohol, and cause sleepiness or electrolyte disturbances in the blood. The detergents contained inside can be caustic causing pharyngeal and esophageal burns, occasionally so severe that children developed respiratory distress and required intubation. Even without ingestion, broken pods can cause damage to sensitive tissue and are blamed for a substantial number of pediatric ocular (eye) burns 4. Despite awareness of these dangers and package labeling detailing potential harms, they continue to be the frequent subject of calls to Poison Control Centers. These cases highlight the need for parents to be aware of the nature and location of potentially toxic substances in their home and ensure that all laundry and detergent pods are safely stored up, away, and out of the reach of children.
Author: Evan Bradley MD, PhD

This year’s International Overdose Awareness Day (August 31st) marks an important milestone for the UMass Memorial Division of Medical Toxicology – one year since the launch of the Overdose Prevention Fund. In an effort to express our appreciation for those who have already pledged their support to the Fund’s mission of eradicating overdoses, we hung up our stethoscopes and reflected on the past 12 months.
In the past year, the Overdose Prevention Fund has:
- supported two studies on fentanyl detection after opioid overdose
- provided experts to community organizations, like the Auburn Elks, to discuss naloxone use
- sent toxicologists to the Sutton Middle School to talk about opioid addiction and drugged driving
- funded summer research assistantships for medical students interested in addiction medicine
- conducted research on naloxone dosing after overdose, biosensor profiles after opioid overdose, and outcomes after ED-based substance use evaluations
- accelerated our collaboration between emergency physicians, medical toxicologists, and outpatient providers in innovative patient care.
We are so grateful for your support of the Overdose Prevention Fund, yet there is much work to be done.
- How do we prevent opioid addiction before it occurs?
- How do recognize opioid tolerance and cravings among our patients?
- How do we optimize care for patients who present to the Emergency Department after overdose?
- How do we reach out to children and adolescents about opioids?
We’re rolling up our sleeves. To support our mission, select Overdose Prevention Fund from the dropdown menu here.
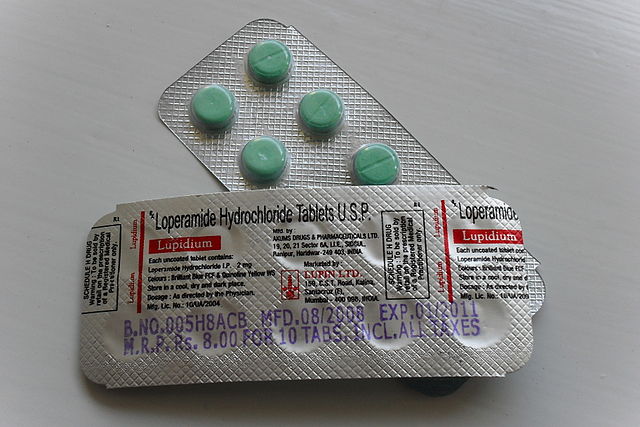
WHAT IS LOPERAMIDE?
Loperamide (known in the United States as the over-the-counter medication Imodium) was discovered in 1969, and is used world-wide as an anti-diarrheal medications. Recently there have been a significant increase in cases of cardiac arrest associated with taking large doses of loperamide, either in attempt to control the diarrhea associated with opioid withdrawal, or in an attempt to get high. Loperamide is a weak mu-receptor agonist (similar to other opioids). This problem has now garnered national attention with the Atlantic article published in April.
HOW DOES LOPERAMIDE WORK?
Loperamide binds primarily to the peripheral mu- receptors located in the GI tract to slow gut motility and reduce the episodes of diarrhea… In essence, loperamide uses the undesired side-effect of opioid-associated constipation to an advantage. However, unlike oxycodone, morphine or other opioid medications, loperamide has a large chemical side chain which prevents the drug from crossing the blood-brain barrier. As a result, loperamide does not cause the typical response to opioid medications – namely analgesia, euphoria, altered mental status, and respiratory depression. Typical adult doses are 2mg, and should not exceed 16mg in a 24 hour period.
WHY IS LOPERAMIDE DANGEROUS?
The chemical structure of loperamide is very similar to that of methadone with two benzene rings and a neighboring ketone group bound to a single carbon. Given their similar structures, it is thought that loperamide also has similar effects to methadone on cardiac potassium channel activity (specifically the human ether-a-go-go potassium channel) which can delay cardiac repolarization in high concentrations . Delayed repolarization prolongs the QT interval and can cause cardiac arrhythmias (such as torsades de pointes) which may be fatal. Loperamide abuse either in the setting to alleviate opioid withdrawal symptoms or in an attempt to get high both have both resulted in cardiac arrests. Doses as low at 80mg have proven to be fatal.
Authors: Matthew Griswold, MD

One of the toxicologic mysteries that repeatedly percolates through the news cycle describes the alleged murder of a Russian whistleblower, Alexander Perepilichnyy, in 2012. Suspicion arose after traces of Gelsemium elegans were supposedly found in the victim’s stomach. Allegedly, the “super grass” was mixed into the sorrel soup Perepilichnyy enjoyed prior to his death.
Yikes… where did the idea come from?
Yes! A Chinese tycoon, Long Liyuan, was allegedly murdered after Gelsemium elegans was mixed into his cat stew in 2011. Two men who shared the stew with Long were also hospitalized but survived. Additionally, three workers who mistakenly drank a liquor containing Gelsemium elegans died in 2016.
What is this “super grass”?
Gelsemium elegans, also known as “heartbreak grass”, grows in Southeast Asia has been used for medicinal purposes as a topical agent for things like eczema, fungal infections, hemorrhoids and leprosy. Gelsemium elegans has been used for its sedative properties to treat anxiety and pain. Ingestion, however, can be lethal. There have been several case reports of Gelsemium toxicity, often from the patients mistaking it for another, more benign plant. Many cases of ingestion resulted in death. In hill tribes in Asia, Gelsemium elegans is known as successful means of suicide.
What happens if you ingest a toxic dose?
Some patients that have been exposed to Gelsemium species survived, but showed signs of toxicity upon ingestion, the most common being dizziness, followed by other signs such as weakness, vomiting, seizures and coma. The toxins, gelsemine and gelsenicine, block transmission at the neuromuscular junction.
Since convulsions have been prevented with benzodiazepines and pentobarbital, yet potentiated by reserpine, investigators speculate that alkaloids from Gelsemium elegans act at GABA receptors. From cases of toxic ingestion and animal studies, it is thought that death occurs from respiratory failure via central inhibition of respiratory drive.
What’s the treatment of a toxic ingestion?
Respiratory support is the keystone of treatment of Gelsemium elegans toxicity. Some patients will require intubation. Death after ingestion of a lethal dose occurs quite rapidly, usually within an hour, therefore medical attention is emergent.
What a terrible plant!
Well actually, alkaloids isolated from Gelsemium species have showed some anti-tumor properties in studies of ovarian and colon cancers. Therefore, this super grass has potential in the development of oncological treatments.
Any pop culture references?
Gelsemium sempervirens (relative of Gelsemium elegans) has been featured on the second season of the “Outlander”, where it was referred to as “yellow jasmine” and was used in assisted suicide. Gelsemium sempervirens acts similarly to nicotine and coniine
But will I ever see this? It doesn’t even grow in the US…
It is unlikely that you’ll encounter a case of Gelsemium elegans poisoning in clinical practice. However, Gelsemium sempervirens is being sold on eBay as plant matter and pills where it is advertised (without supporting evidence) as an alternative remedy for anything from pain and anxiety to asthma and whooping cough. Therefore, yellow jasmine toxicity could be coming to the emergency department near you!
Author: Dr. Svetlana Fridlyand
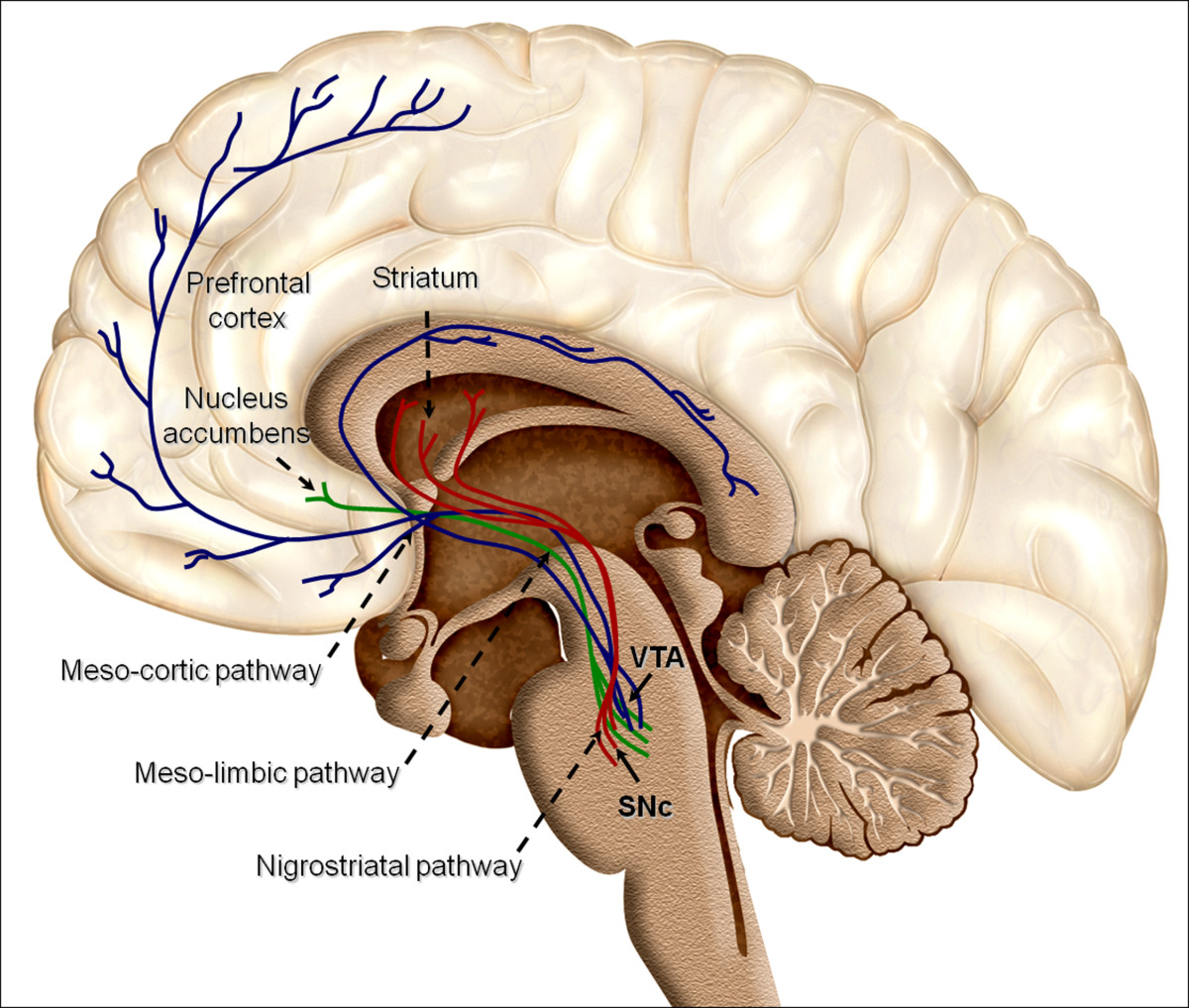
We are winging it home from San Juan, Puerto Rico, after the American College of Medical Toxicology Annual Scientific Meeting. I am lucky enough to be sitting next to Dr. Christina Hernon, whose presentation, “Twisted: Understanding the Neural Pathways of Addiction” shined a light on an area of increasing importance in medical toxicology.
If you’ve never met Dr. Hernon, one of her superpowers is distilling complex ideas into comprehensible bits. I had the chance to ask her about her favorite take-home messages from the lecture.
Our understanding of addiction is evolving rapidly. Historically, laypeople, family members, and even doctors pointed the finger at the addicted patient, assuming some sort of social flaw, moral failing, or lack of willpower drove the addiction and prevented recovery. But by understanding the biology underlying addiction (the pathways, structures, neurotransmitters, transporters, etc.) that are ultimately overtaken or hijacked in addiction, the more we can respect the drive towards addiction and the complexity of recovery.
2. First exposures are critically important.
A recent MMWR article indicated that a 10-day opioid prescription leads to a 1 in 5 chance that the patient will still be on an opioid one year later. What impressed me the most from the emergency physician point-of-view is that the same article had a graph showing that even a 1-day prescription for an opioid leads to a 6% chance that the patient is still on an opioid at one year. Here’s an example drawing from these data. If an emergency physician writes 5 opioid prescriptions per week for a 1-day supply, then in one year, 15.6 of that physician’s patients will still be taking opioids. We must be mindful of the long-term impact of opioid prescriptions, including those of short duration.
3. Humans are driven towards homeostasis.
To find homeostasis, we balance sensory input of two main categories. The first is exteroception, or sensing the physical world. Think proprioception or discriminatory touch. The second is interoception, which includes thirst, hunger, temperature, pain, itch, and tickle, among others. Some of the sensations of withdrawal overlap with these interoceptive inputs. As a result, the drive for homeostasis effectively includes a drive to alleviate the symptoms of withdrawal that rivals the drive to eliminate feelings of thirst, hunger, and pain.
Understanding the biology of addiction is key to destroying any lingering shame or stigma faced by our patients with addiction.
Authors: Dr. Christina Hernon and Dr. Kavita Babu
UMassTox
April 3, 2017
Categories: Addiction, Medical Audience
Tags: addiction, Biopsychosocial, Interoception, Opioid, Prescription, Research, Stigma, Toxicology, Withdrawal
Comments

As you prepare for or recover from a storm, think about keeping your family safe from carbon monoxide poisoning. Carbon monoxide is an odorless and colorless gas that causes flu-like symptoms (headache, nausea, fatigue), chest pain, and fainting (syncope). People who are exposed to a high concentration of carbon monoxide are at risk for brain injury and death. Furnaces, heaters, generators, gas stoves, charcoal grills, cars, and trucks are all important sources of carbon monoxide.
Why worry about carbon monoxide and big storms?
During every big storm, we will hear about families being injured or killed by carbon monoxide. This poisoning is preventable.
We worry about GENERATORS during big storms. When people lose power, they start up their generators. People even use GAS STOVES inside when they’re cold. Both generators and gas stoves produce significant quantities of carbon monoxide.
We also regularly hear about cases in which someone is poisoned by carbon monoxide while sitting in a running car with a BLOCKED TAIL PIPE. When the tail pipe is blocked with snow or mud, carbon monoxide backs up into the passenger compartment. Unfortunately, children have been killed this way (keeping warm while mom or dad shovels snow). This happens FAST! In one experiment, dangerous levels of carbon monoxide accumulated inside the passenger compartment after less than 2 minutes of running the engine with a clogged tail pipe.
How can I keep my family safe?
- Have working carbon monoxide detectors
- Never use a generator within 20 feet of your living space
- Keep the generator more than 20 feet from vents, windows, and doors
- Never run a generator in the attached basement or garage of your home
- Do not use a portable gas stove or charcoal grill inside
- Never run a car/truck with a blocked tailpipe
What should I do now to prepare (before the storm)?
Since some carbon monoxide detectors are powered by electricity, make sure you have a battery-operated or battery back-up model. You can also keep a battery-operated carbon monoxide detector with your generator, so you’ll always have one ready to go.
Some people run a generator inside because they are worried about it being stolen. Think now about where you will put your generator. Make sure that spot keeps your generator far enough from the house, and consider using a cable lock to keep it secure there.
Share this information with your family and friends. Let’s make sure no one dies of carbon monoxide poisoning during this next winter storm.
Authors: Dr. John Broach and Dr. Kavita Babu
Links for further reading:
An awesome resource from the CDC on Carbon Monoxide Poisoning Prevention
Information from the National Fire Protection Association – make sure to watch the video at the bottom of the page
A description of how it takes for a car to fill with carbon monoxide when the tail pipe is clogged

Toxicology has been front and center in the media recently with the high-profile assassination of Kim Jong-nam ( the estranged half-brother of North Korean Supreme Leader Kim Jong-un, at the airport in Kuala Lumpur. Preliminary reports from the Malaysian government suggest that VX nerve agent was the murder weapon. VX is a weaponized version of a class of pesticides called organophosphates (OPs). These agents disrupt the function of the autonomic nervous system.
The auto what? That doesn’t sound very exciting…
It isn’t, but that’s the point. The autonomic nervous system governs vital functions such as blood pressure, heart rate, temperature regulation, and digestion, and it does its job without us having to think much about it. When VX comes along, however, it totally mucks with one of the system’s key neurotransmitter chemicals, acetylcholine.
OK, that’s more interesting. But why is VX bad?
VX binds to and irreversibly inactivates an enzyme called acetylcholinesterase (AChE). AChE breaks down acetylcholine, making sure that acetylcholine is present in appropriate amounts – not too much, not too little. By inactivating AChE, you get a massive buildup of acetylcholine, which sends the organs that receive acetylcholine nerve signals into overdrive.
The first symptom might be some sweating and muscle twitching. Then some runny nose or tearing and shortness of breath, which progresses to vomiting and diarrhea. Basically, acetylcholine makes organs produce LOTS of extra secretions (think mucus, tears, saliva, gastric juices, sweat…). It makes air passages spasm and fill up with mucus – think body fluids pouring out of every orifice. Muscles become paralyzed, and VX-poisoned patients start to drown in their own secretions and develop seizures, slip into a coma, and then die.
Wait, I think I remember VX… like in that awesome 90s movie, The Rock?!?
Kinda. VX was the weapon that the bad guys stockpiled on Alcatraz in that classic movie. If you haven’t watched it (recently), you should probably go watch it now. Now that you’ve (re-)watched it, you know that Nicholas Cage and Sean Connery were trying to stop the bad guys from releasing the VX over San Francisco and killing millions of civilians. They went a little Hollywood with it (in real life, faces don’t melt off), but the writers had the right idea.
Whoa, so people could really use nerve agents for terrorism?
Unfortunately, yes. But there are differences between the various agents. The “V” series nerve agents are not very volatile, meaning they don’t evaporate very easily. Therefore, the primary method of exposure to VX is through skin contact. There are other OP nerve agents, such as Sarin which has been used in terrorist attacks and against civilians (e.g., Tokyo subway 1995; Ghouta, Syria 2013)
Is VX legal?
Nope. It was classified as a weapon of mass destruction by UN Resolution 687, and the Chemical Weapons Convention of 1993 made production and stockpiling of VX illegal. That apparently doesn’t stop these assassins though…
What are other chemicals that act like VX?
VX belongs to a group of chemical nerve agents known as the “V agents”. Other members of this group are VE, VG (AKA tetram), and VM.
There are other groups of nerve agents that act similarly, and they are separated from one another by their volatility (or their ability to be liquids at room temperature). The “G series” is made up of sarin, soman, tabun, and cyclosarin. The GV series contains the deadly “Novichok Agents” and GV.
Why did anyone make VX in the first place?
VX was discovered in 1952 by a company in Great Britain. They were actually looking to develop pesticides, and while VX is a very effective pesticide, it is also incredibly dangerous to humans.
If I get exposed, do I have to stab myself in the heart, a la Nicholas Cage?
Absolutely (just kidding). The antidote consists of 2 medicines, atropine and pralidoxime, which can be injected into either a vein or a muscle. Atropine binds to the same target as acetylcholine in the nervous system, and prevents the built-up acetylcholine from putting organ systems into overdrive. The goal of its use is to dry up secretions in the airway so poisoned patients don’t suffocate on their secretions or develop seizures. Pralidoxime, if given early in nerve agent poisoning, pushes VX and other OPs off of AChE, reviving the enzyme and allowing it to work again. Time is key though – if you wait too long, OPs bind so tightly to AChE is broken until your body can regenerate it (which takes weeks).
Authors: Dr. Katharine Devin-Holcombe and Dr. Jeffrey Lai
Links for further reading:
The Poison Review description of VX and the assassination
CNN description of the assassination
CDC page on VX effects
Newsweek article with quotes from Dr. Paul Wax and Dr. John Trestrail

Recently, we’ve had several adults report using Sassafras (or “Sass”) to get high at concerts and in social situations. In researching sassafras, this is what we learned.
Like Sassafras… the plant?
Sort of. Safrole (4-allyl-1,2-methylenedioxy-benzene) is a phenylpropene oil derived from sassafras plants (typically root bark and fruits). Safrole can be isolated from camphor oil, and can also be synthesized from catechol. Naturally-occurring sassafras oil contains approximately 80% safrole. However, safrole is prohibited from inclusion in food products since the 1960s due to concerns about its carcinogenicity (linked to liver cancer). Safrole is used in the production of insecticides and fragrance.
Why would anyone use safrole?
Safrole is also a precursor for MDA (methylenedioxyamphetamine) and MDMA (Ecstasy). In one 2015 drug lab raid, the Royal Canadian Mounted Police seized 1500 kg of sassafras oil. News media reported that those 1500 kg could ultimately be converted into 4.2 million tablets of MDMA . Sassafras has been around for years; user reports go back to at least 2010. The terms Sass or Sassafras are sometimes used to refer to MDA itself, and users may refer to Sassafras as “natural Ecstasy.”
Can safrole make you sick?
Clear reports of safrole-induced illness are lacking. However, MDA and MDMA toxicity are well-described, and can include agitation, high temperature (hyperthermia), seizures, abnormal cardiac rhythms (dysrhythmias), and death . Since drug names can be used inaccurately, Sassafras cannot be linked to an actual drug without rigorous drug testing. In one death reported in the media, Sassafras was reported by witnesses as the culprit agent.
Is safrole legal?
Safrole is a List I Chemical in the United States. A chemical is designated as List I by the DEA if in addition to legitimate uses, it is used in manufacturing a controlled substance or is important to the manufacture of a controlled substance.
Right now, “It is unlawful for any person knowingly or intentionally to possess or distribute safrole, knowing, or having reasonable cause to believe, the safrole will be used to manufacture MDMA.”
Will we be seeing more of this?
We don’t know yet. But we’ll be looking for it. Any more information on Sassafras? Feel free to Tweet at us @umasstox.
Authors: Dr. Albert Conicella and Dr. Kavita Babu
Links for further reading:
A US Department of Justice advisory on safrole and sassafras oil.
A description of the role sassafras may have played in singer Scott Weiland’s death.
A news story on a death attributed to sassafras in Chicago.
A DEA description of what makes a List I Chemical.

On August 31, 2016, the Drug Enforcement Administration (DEA) temporarily placed mitragynine and 7-hydroxymitragynine into Schedule I status, then withdrew the intent to schedule in favor of a public comment period until December 1, 2016. The status of Kratom remains undetermined. Mitragynine and 7-hydroxymitragynine are the active alkaloids found in Kratom, a plant-derived product that has recently been used by patients to self-treat opioid withdrawal and addiction. Two strong camps are advocating for and against scheduling with patient safety in mind.
History
Kratom is derived from a plant native to Southeast Asia. For centuries, Kratom was used by manual laborers in countries like Thailand to mitigate the boredom and fatigue of working outside in the midday sun. At low doses, Kratom (usually chewed or taken as a tea) caused stimulant effects; at higher doses, an analgesic effect occurred. Kratom was made illegal in Thailand in 1943. Accounts of Kratom addiction entered the medical literature as early as the 1960s, with subsequent accounts describing a syndrome of malaise, apathy, and facial hyperpigmentation among the “Kratom eaters.”
This Century
Circa 2005, Kratom became increasingly available over the Internet as plant material, tea, and extracts. On forums like Bluelight, chronic opioid users began describing the use of Kratom to decrease their opioid tolerance during “opioid holidays,” and to manage withdrawal symptoms.
Adverse Events
Reports of adverse events followed, including hepatic injury, seizures, and deaths. However, the Kratom in several of these cases was contaminated with other agents. In one series of deaths in Europe, Kratom under the brand name “Krypton” was adulterated with a pharmaceutical, O-desmethyltramadol, an opioid analgesic with potentially deadly side effects in overdose.
The Kratom Prohibition Camp
Arguing for placement in Schedule I, the DEA entry in the Federal Register contained the following:
Kratom does not have an approved medical use in the United States and has not been studied as a treatment agent in the United States. Kratom has a history of being used as an opium substitute in Southeast Asia. Kratom has also been used to self-treat chronic pain and withdrawal symptoms from opioid use. Especially concerning, reports note users have turned to kratom as a replacement for other opioids, such as heroin. In the United States, kratom is misused to self-treat chronic pain and opioid withdrawal symptoms, with users reporting its effects to be comparable to prescription opioids.
The entry also notes that there are no pending Investigational New Drug (IND) applications pending for Kratom – an initial step required for formal study.
The Free-the-Kratom Camp
Advocates for patients with addiction have responded to the scheduling of Kratom with frustration. Many perceive Kratom as safer than pharmaceutical opioids, citing the limitations of drug treatment availability in describing the continued need for Kratom availability. Others have pointed to a knee-jerk response to new opioids in the midst of the opioid epidemic as the reason for the momentum in scheduling this drug.
The Bottom Line
Like other opioids, the potential for Kratom addiction and complications of use exist. However (from my perspective), Kratom has been used for centuries, with a handful of reported adverse events. The funds and effort required to enforce a Kratom ban would be better spent elsewhere. Yet, consumers must remain wary regarding Kratom safety, since there is no guarantee that the purchased Kratom today resembles the Kratom of old. Adulteration with pharmaceuticals and laboratory grade agents (including 7-hydroxymitragynine) have been reported, and many Kratom products are noted to be “enhanced.” Without safeguards on mitragynine and 7-OH-mitragynine concentrations, patients are at increased risk for adverse events. Limits on mitragynine and 7-OH-mitragynine concentration seem more warranted and reasonable than Schedule I status.
Author: Dr. Kavita Babu
Links for Further Reading
An awesome podcast featuring Dr. Alicia Lydecker on Kratom:
Smart Drug Smarts
A discussion of the scheduling debate with Drs. Boyer and Babu:
NY Times: Room for Debate
A recent publication from our group on the adulteration of Kratom:
Recent publication from our group









